使用瞬时保护氨基酸的反向肽合成
Inverse Peptide Synthesis Using Transi����ent Protected Amino Aci�������ds
Tao Liu,Zejun �������Peng,Manting Lai,Long Hu, andJunfeng Zhao*
Cit�������e this:J. Am. Chem. Soc.2024, 146, 6, 4270–4280
Publication Date:February 5, 2024
//doi.org/10.1021/jacs.4c00314
使用瞬时保护氨基酸的反向肽合成
主题:肽类药物的化学合成技术
背景:
过去三十年内,肽类药物迎来了迅速发展。
大部分肽药物通过化学合成方式制造,尽管也有一部分是生物生产的。
传统方法的局限:
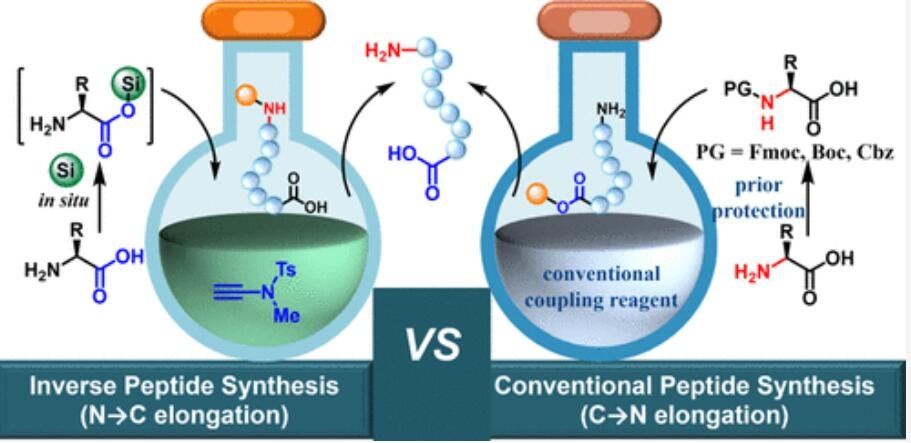
传统C→N肽化学合成的过程包括$������α-$氨基酸的氨基保护、羧基激活、与肽链游离氨基的氨解反应以及N-末端去保护。这种方法因为强制使用α保护基而导致步骤和原子使用效率低下。
市场需求:
随着肽类药物市场的迅猛增长,人们迫切需要成本低廉且环境友好的生产策略。
研究进展:
提出使用未保护氨基酸的反向����肽化学合成作为理想策略,但60多年来由于严重的外消旋/表里异构化问题而未能成功。
技术突破:
本研究通过使用ynamide偶联试剂和瞬时保护策略,成功地解决了N→C肽链延长过程中的外消旋��������/表里异构化问题。
实现了激活、瞬时保护、氨解和原位去保护步骤的一锅合成,有效利用了未保护氨基酸作为起始物料。
应用潜力:
该策略的稳健性通过肽活性药物成分的合成得到证明。
适用于片段缩合和反向固相肽合成,与绿色溶剂的兼容性提高了其在大规模生产中的应用潜力。
结论:
这项研究提出了一种成本效益高、操作简便且环境友好的肽类制备新方法。
Peptide therapeutics have experienced a rapid resurgence over the past three decades. While a few peptide drugs are biologically produced, most are manufactured via chemical synthesis. The cycle of prior protection of the amino group of an α-amino acid, activation of its carboxyl group, aminolysis with the free amino group of a growing peptide chain, and deprotection of the N-terminus constitutes the principle of conventional C → N peptide chemical synthesis. The mandatory use of the Nα-protecting group invokes two additional operations for incorporating each amino acid, resulting in poor step- and atom-economy. The burgeoning demand in the peptide therapeutic market necessitates cost-effective and environmentally friendly peptide manufacturing strategies. Inverse peptide chemical synthesis using unprotected amino acids has been proposed as an ideal and appealing strategy. However, it has remained unsuccessful for over 60 years due to severe racemization/epimerization during N → C peptide chain elongation. Herein, this challenge has been successfully addressed by ynamide coupling reagent employing a transient protection strategy. The activation, transient protection, aminolysis, andin situdeprotection were performed in one pot, thus offering a practical peptide chemical synthesis strategy formally using unprotected amin�����o acids as the starting material. Its robustness was exemplified by syntheses of peptide active pharmaceutical ingredients. It is also amenable to fragment condensation and inverse solid-phase peptide synthesis. The compatibility to green solvents further enhances its application potential in large-scale peptide production. This study offered a cost-effective, operational convenient, and environmentally benign approach to peptides.
过去三十年里,肽类药物经历了迅速的复兴。虽然少数肽药物是通过生物方式生产的,但大多数是通过化学合成制造的。α氨基酸的氨基保护、其羧基激活、与生长中的肽链的游离氨基进行氨解以及N-末端的去保护构成了传统C→N肽化学合成的原理。$Nα-$保护基的强制使用使得每加入一个氨基酸就需要进行两个额外操作,导致较差的步骤和原子经济性。肽类药物市场的迅猛需求促使人们需要成本效益高和环境友好的肽制造策略。使用未保护的氨基酸进行反向肽化学合成被提出为一种理想而吸引人的策略。然而,由于N→C肽链延长过程中严重的外消旋/表里异构化,这一策略60多年来一直未能成功。在这里,通过使用ynamid������e偶联试剂采用瞬时保护策略,成功地解决了这一挑战。激活、瞬时保护、氨解和原位去保护在一锅中完成,从而提供了一种实际上使用未保护氨基酸作为起始物料的肽化学合成策略。它的稳健性通过肽活性药物成分的合成得到了体现。它也适用于片段缩合和反向固相肽合成。与绿色溶剂的兼容性进一步增强了其在大规模肽生产中的应用潜力。这项研究提供了一种成本效益高、操作便利和环境友好的肽类制备方法。
使用瞬时保护氨基酸的反向肽合成
Inverse Peptide Synthesis Using������� Transient Protected Amino Acids
Tao Liu,Zejun Peng,Manti�����������ng Lai,Long Hu, andJunfeng Zhao*
Cite this:J. Am. Chem. Soc.2024, 146,������� 6, 4270–4280
Publication Date:February 5, 2024
//doi.org/10.1021/jacs.4c00314
JACS | 使用瞬时保护氨基酸的反向肽合成 Inverse Peptide Synthesis Using Transient Protected Amino Acids Tao Liu,Zejun Peng,Manting Lai,Long Hu, andJunfeng Zhao* Cite this:J. Am. Chem. Soc.2024, 146, 6, 4270–4280 Publication Date:February 5, 2024 //doi.org/10.1021/jacs.4c00314 Copyright © 2024 American Chemical Society Request reuse permissions







 15vip太阳集团(中国)有限公司官网入口
15vip太阳集团(中国)有限公司官网入口
 地址:
地址:
 Email: info@songxia-06.com
Email: info@songxia-06.com
 总机:025-58361106-801
总机:025-58361106-801
